Definition, impacts on human health and environment
Definition
Persistent Organic Pollutants (POPs) are organic chemical substances, i.e., they are carbon-based. They possess a particular combination of physical and chemical properties such that, once released into the environment, they:
- remain intact for exceptionally long periods of time (many years)
- become widely distributed throughout the environment as a result of natural processes involving soil, water and, most notably, air
- accumulate in the fatty tissue of living organisms including humans, and are found at higher concentrations at higher levels in the food chain
- are toxic to both humans and wildlife.
Secretariat of the Stockholm Convention: http://chm.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx
Initially, the Stockholm Convention recognized twelve POPs as causing adverse effects on humans and the ecosystems. They are categorized in 3 groups:
- Pesticides
- Industrial chemicals
- By-products / unintentional products
In the years 2009 to 2017, sixteen more chemicals have been added to the list and this process is still ongoing. There are more chemicals under review and chemicals can still be proposed for review. A full list of chemicals is provided on the website of the Convention: http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx
The Stockholm Convention lists the POPs in three Annexes with different management strategies:
- Annex A (Elimination): Parties must take measures to eliminate the production and use of the chemicals listed under Annex A. Specific exemptions for use or production are listed in the Annex and apply only to Parties that register for them.
- Annex B (Restriction): Parties must take measures to restrict the production and use of the chemicals listed under Annex B in light of any applicable acceptable purposes and/or specific exemptions listed in the Annex.
- Annex C (Unintentional production): Parties must take measures to reduce the unintentional releases of chemicals listed under Annex C with the goal of continuing minimization and, where feasible, ultimate elimination.
More information on the Stockholm Convention and on POPs in general are available on the website of the Convention: http://chm.pops.int/
The full text of the Stockholm Convention is available here: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx
The full text of the 1998 Protocol on Persistent Organic Pollutants, including the amendments adopted by the Parties on 18 December 2009, is available on the UNECE website: https://www.unece.org/fileadmin/DAM/env/lrtap/full%20text/ece.eb.air.104.e.pdf
As the Stockholm Convention, the UNECE 1998 Protocol on Persistent Organic Pollutants lists the POPs in three Annexes with different management strategies:
- Annex I: substances scheduled for elimination
- Annex II: substances scheduled for restrictions on use
- Annex III: substances referred to in article 3, paragraph 5 (a) such as PAHs, Dioxins/furans, hexachlorobenzene and PolyChlorinated Biphenyls (PCBs) when formed and released unintentionally from anthropogenic sources. The annual emissions of each of the substances have to be reduced, from the level of the emission in a reference year set in accordance with that annex by taking effective measures, appropriate in its particular circumstances and the reference year for the obligation.
Impacts on Human Health and Environment
The life-time of POPs can range from several days in the atmosphere up to years or decades in soils and biota. Due to their high persistency, POPs can travel long distances before decomposing. Most POPs are hydrophilic and are hence stored in the fat tissue of organisms. They can accumulate in food chains and are therefore a special threat to predatory animals and humans (Jones & de Voogt, 1999).
For birds, seals and whales a correlation between the POP levels in their tissue and a decline in population could be shown. Because of the large number of different POPs, which all accumulate in one organism, the effects of the distinct chemical are difficult to predict. POPs have further been connected to reproductive impairment, damage of the immune system and cancer. Many POPs are accidental by-products of the synthesis of other chemicals or are produced for industrial use or as agrochemicals (Jones & de Voogt, 1999). A well-known example are organochlorine pesticides (e.g. DDT). A further class of POPs are polycyclic aromatic hydrocarbons (PAHs), which can be emitted by combustion processes. They are natural compounds of fossil fuels or can be formed from precursors during combustion. Dioxins and polychlorinated dibenzofurans, also referred to as Dioxins and Dioxin-Like Compounds (DLCs), are a special subgroup of PAHs. They are polyhalogenated PAHs, which are especially toxic. Since they are highly toxical a lot of research focuses on the formation and abatement of DLCs. Yet the same processes apply for the formation and prevention of all PAHs. Another famous example of POPs are PCBs, PolyChlorinated Biphenyls. These compounds are or have been used in industry as heat exchange fluids, in electric transformers and capacitors, and as additives in paints, carbonless copy paper, and plastics. Of the 209 different types of PCBs, 13 exhibit a dioxin-like toxicity. Their persistence in the environment corresponds to the degree of chlorination, and half-lives can vary from 10 days to one-and-a-half years.
(cf. http://chm.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx)
References of this chapter:
Jones, K. C. K. C., & de Voogt, P. (1999). Persistent organic pollutants (POPs): state of the science. Environmental Pollution, 100(1-3), 209–221. http://doi.org/10.1016/S0269-7491(99)00098-6
Origin and Formation Processes
In an industrial context, POPs have two main sources. They can be formed in chemical processes including combustion, either on purpose or as by-products, or they can be introduced in a company by POP containing raw materials. This for example the case in waste incineration plants, or in food processing industries. Due to the high number of POPs, it is not possible to present the formation processes for all substances in detail. A short overview for several important groups and subgroups of substances is provided below.
Formation of PCDD/PCDF, PCB and HCB and Poly Aromatic Hydrocarbons (PAH)
Polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF), polychlorinated biphenyls (PCB) and hexachlorobenzene (HCB) are unintentionally formed in industrial-chemical processes, such as chemical manufacture, and thermal processes, such as waste incineration. PCDD/PCDF are the only by-product POPs whose mechanism of formation has been studied extensively in combustion-related processes and to a lesser extent in non-combustion-related chemical processes; even so, the mechanisms and exact formation conditions are not fully resolved. It is clear that the predominant mechanism or pathway can vary from process to process so that different factors become controlling and there is no universal controlling factor.
There is far less information as to the formation of PCB and HCB, especially in combustion processes. Since there are similarities in the structure and occurrence of PCDD/PCDF, PCB and HCB, it is usually assumed that, with the exception of oxygen containing species, those parameters and factors that favour formation of PCDD/PCDF also generate PCB and HCB.
Carbon, oxygen, hydrogen and chlorine, whether in elemental, organic or inorganic form, are needed. At some point in the synthesis process, whether present in a precursor or generated by a chemical reaction, the carbon must assume an aromatic structure. There are two main pathways by which these compounds can be synthesized: from precursors such as chlorinated phenols or de novo from carbonaceous structures in fly ash, activated carbon, soot or smaller molecule products of incomplete combustion. Under conditions of poor combustion, PCDD/PCDF can be formed in the burning process itself.
Variables known to impact the thermal formation of PCDD/PCDF include:
- Technology: PCDD/PCDF formation can occur either in poor combustion or in poorly managed post-combustion chambers and air pollution control devices. Combustion techniques vary from the very simple and very poor, such as open burning, to the very complex and greatly improved, such as incineration using best available techniques;
- Temperature: PCDD/PCDF formation in the post-combustion zone or air pollution control devices has been reported to range between 200°C and 650°C; the range of greatest formation is generally agreed to be 200 – 450°C, with a maximum of about 300°C;
- Metals: Copper, iron, zinc, aluminium, chromium and manganese are known to catalyse PCDD/PCDF formation, chlorination and dechlorination;
- Sulphur and nitrogen: Sulphur and some nitrogen-containing chemicals inhibit the formation of PCDD/PCDF, but may give rise to other unintended products;
- Chlorine must be present in organic, inorganic or elemental form. Its presence in fly ash or in the elemental form in the gas phase may be especially important;
- PCB are also precursors for the formation of PCDF.
For the formation in industrial-chemical processes, as with thermal processes, carbon, hydrogen, oxygen and chlorine are needed. PCDD/PCDF formation in chemical processes is expected to be favoured by one or more of the following conditions:
- Elevated temperatures (>150°C);
- Alkaline conditions;
- Metal catalysts;
- Ultraviolet (UV) radiation or other radical starters.
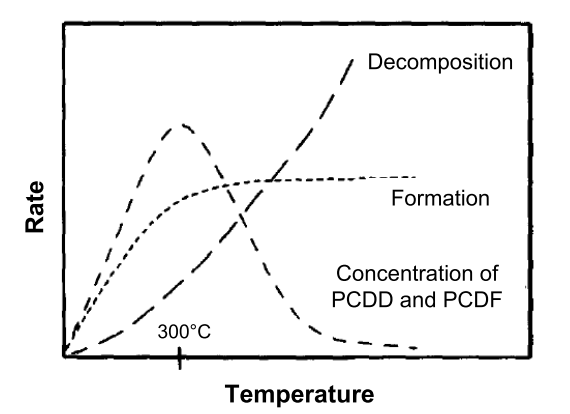
Figure 1. The dualistic principle of PCDD/F formation and destruction. (Wehrmeier et al., 1998)
References of this section
The full section on the Formation of PCDD/PCDF, PCB and HCB refers to:
Secretariat of the Stockholm Convention on Persistent Organic Pollutants (2008). Guidelines on best available techniques and provisional guidance on best environmental practices relevant to Article 5 and Annex C of the Stockholm Convention on Persistent Organic Pollutants
http://chm.pops.int/Implementation/BATBEP/BATBEPGuidelinesArticle5/tabid/187/Default.aspx
Other References:
Wehrmeier, A., Lenoir, D., Schramm, K.-W., Zimmermann, R., Hahn, K., Henkelmann, B., & Kettrup, A. (1998). Patterns of isomers of chlorinated dibenzo-p-dioxins as tool for elucidation of thermal formation mechanisms. Chemosphere, 36(13), 2775–2801. http://doi.org/10.1016/S0045-6535(97)10236-3
Abatement Technologies
As for the formation processes, abatement technologies may vary due to the differences between the POP substances. Nevertheless, the following section summarizes some important abatement technologies. More detailed information is provided in:
- UNECE Guidance document on Best Available Techniques to Control Emissions of Persistent Organic Pollutants from Major Stationary Sources:
https://www.unece.org/environmental-policy/conventions/envlrtapwelcome/guidance-documents-and-other-methodological-materials/protocol-on-pops.html - BAT (Best Available Techniques) and BEP (Best Environmental Practice) Guidance by the Secretariat of the Stockholm Convention:
http://chm.pops.int/Implementation/BATandBEP/Guidance/Overview/tabid/5121/Default.aspx - The abatement of POPs is covered in several sector specific BREF (Best Available Techniques Reference) Documents. All BREFs can be downloaded from the IPPC JRC website:
http://eippcb.jrc.ec.europa.eu/reference/
The Guidance document of the UNECE lists five general measures that may be used to reduced POP emissions:
(a) Replacement of feed materials which are POPs or where there is a direct link between the composition of the raw-materials and POPs emissions from the source;
(b) Best environmental practices such as good housekeeping, preventive maintenance programmes, or process changes such as closed systems (for instance in cokeries or use of inert electrodes for electrolysis);
(c) Modification of process design to ensure complete combustion, thus preventing the formation of persistent organic pollutants, through the control of parameters such as incineration temperature or residence time;
(d) Methods for flue-gas cleaning such as thermal or catalytic incineration or oxidation, dust precipitation, adsorption;
(e) Treatment of residuals, wastes and sewage sludge by, for example, thermal treatment or rendering them inert.
The document further lists specific POP reducing measures for several industries and provides important data and parameters.
Primary Measures
(McKay, 2002) proposes the following combination of primary measures for dioxin emission reduction in solid waste incinerations. Other combustion processes are generally less critical with regard to POP-emission limits, because other fuels contain lower amounts of halogenides and the combustion parameters do not enforce the formation of DLCs. Low DLC combustion parameters are:
- Combustion temperatures above 1000°C
- Residence time greater than one second (respectively two seconds at 850°C)
- Excess of oxygen of 3-6% (v/v) (in order to ensure a complete combustion)
- Chamber turbulences represented by a Reynolds number greater than 50,000
- Rapid cooling of the flue gas from 400 to 250°C or below can further decrease dioxin levels (since the de novo syntheses is the major formation path of DLCs)
Such strategies can easily be retrofitted in existing plants and are typically sufficient to gain DLC-emissions below 1 ng(TE)/m3 in waste combustion plants (Vehlow, 2005).
Secondary Measures
Most combustion facilities do not require any secondary measures to meet POP emission limits. Some others, like solid waste incinerations, however, need additional measures to comply with the regulations. Since the de novo synthesis takes place on the surface of particles, a large share of POPs stick to the dust in the flue gas. A large part can therefore be separated with the dust.
Various secondary measures, which are also used to reduce other pollutants, can also affect POP emissions. (McKay, 2002) explains the effects of secondary measures to dioxin emissions in solid waste incinerations. Since dioxins have similar chemical properties than other POPs, the results can be transferred to all POPs and combustion plants. However, the secondary measures are usually implemented to abate other pollutants than POPs. Therefore, it is not possible to account the costs solely to POPs.
Due to their high contamination with POPs and other pollutants, filter ashes and other gas cleaning residues of waste incinerations are classified as toxic. The main problem is therefore not the separation of the POPs from the flue gas, but the treatment of the residues (Vehlow, 2005).
Electrostatic precipitators (ESP)
Electrostatic precipitators are installed to remove particulate matter from the flue gas stream. They separate electrostatic chargeable particles from the flue gas. As mentioned before, a big part of the POPs can be separated herewith, since the POPs often stick to particles. However, lowering the particle emissions does not always result in lower POP emissions. At temperatures between 150 and 300°C new POPs can be formed through the de novo path in the electrostatic precipitator (McKay, 2002). (See Figure 1)
Fabric filters (FF)
Fabric filters are another way to remove particulate matter from the flue gas and therefore to reduce POP emissions.
Dry scrubbers
The neutralising capacity of hydrated lime reduces the content of acid gas constituents (e.g. hydrogen chloride gas, and sulphur dioxide gas) in the flue gas. The temperature of the lime-water injection is usually in the range of 130–150°C. Dry scrubbers are most commonly used in combination with ESPs or FFs. Since an upstream dry scrubber reduces the electrostatic precipitator’s inlet temperature, the de novo synthesis is reduced. Dry scrubber fabric filter combinations have achieved DLC emission reductions greater than 95% in MSWI. (McKay, 2002)
Dry sorbent injections
Activated carbon is used in a dry/semi-dry scrubbing units downstream the particular removal device as a new technique. This has become a standard technique for DCL control in MSW. (McKay, 2002)
Wet scrubber
Wet scrubbers follow the same principals as dry scrubbers. They are more common in MSWI in Europe. DLC emissions bound to particles or in the gas phase can be separated from the flue gas. (McKay, 2002)
Selective Catalytic Reduction (SCR)
SCR was initially developed to reduce NOx emissions. It has also proven to be able to decompose organohalogen compounds, including DLCs. There have been successful trials at MSW and hazardous waste incineration facilities reducing the DLC emissions below 0.1 ng I-TEQ/m3 without any ammonia slip using titanium dioxide catalysts. Some other catalysts, however, might increase DLC emissions. (McKay, 2002)
Combination of scrubber bag filter and activated carbon absorption
The combination of a scrubber and a bag filter with an active carbon injection has shown to be the most effective end-of-pipe measure. The necessary carbon consumption is around 50mg/Nm³ and the filter should be operated below 200°C. (McKay, 2002)
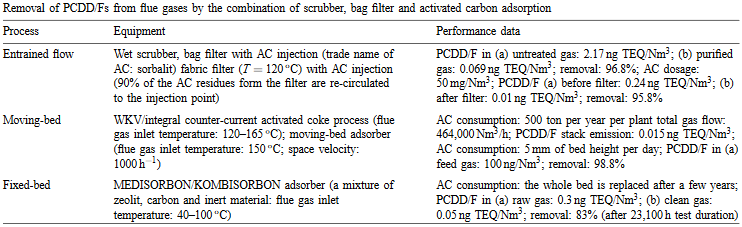
Figure 2: Performance parameters of secondary abatement techniques (McKay, 2002)
The BREF for waste incineration support and complements the techniques described above, not only for POP and for DLCs, but in particular for PCDD and PCDF. The following techniques are mentioned as BAT. Detailed descriptions are provided under:
http://eippcb.jrc.ec.europa.eu/reference/BREF/wi_bref_0806.pdf
- Destruction of PCDD/F using Selective Catalytic Reduction (SCR)
- Destruction of PCDD/F using catalytic filter bags
- Destruction of PCDD/F by re-burn of adsorbents
- Adsorption of PCDD/F by activated carbon injection or other reagents
- Adsorption of PCDD/F in static beds
- Use of carbon impregnated materials for PCDD/F adsorption in wet scrubbers
- Use of carbon slurries in wet scrubbers
The BAT conclusions for Large Combustion plants lists the following techniques as BAT: Detailed descriptions are provided under:
https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1503383091262&uri=CELEX:32017D1442
- Destruction of PCDD/F using Selective Catalytic Reduction (SCR)
- Adsorption of PCDD/F by activated carbon injection in the flue gases
- Rapid quenching using wet scrubbing/flue-gas condenser
References of this section
McKay, G. (2002). Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: Review. Chemical Engineering Journal, 86(3), 343–368. http://doi.org/10.1016/S1385-8947(01)00228-5
Vehlow, J. (2005). Dioxins in Waste Combustion – Conclusions from 20 Years of Research. Bioenergy Australia 2005, 1–10.
