Click on "+" to develop chapter
Definitions and main sources
Molecular nitrogen (N2) is an inert gas that makes up about 80% of the atmosphere and is not dangerous for humans or the environment. Nitrogen, however, can react with oxygen to build nitrogen oxides, also called NOx, like nitrogen oxide (NO), nitrogen dioxide (NO2) and higher orders.
The generic term NOx refers to the sum of nitrogen oxide (NO) and nitrogen dioxide (NO2), expressed as NO2. Nitrous oxide (N2O), a greenhouse gas, is not covered in NOx. The main source for NOx is combustion where primarily NO is formed. NO is then rapidly converted to NO2.
N2O or laughing gas is a nitrogen oxide that is often emitted from natural sources and not always accounted to NOx due to its chemical structure. N2O is sometimes, but not everywhere, subjected to regulations for technical combustion. It can be emitted by fluidised bed combustion and other industrial processes.
NOx emissions contribute to acidification via formation of nitrous acid (HNO2) and nitric acid (HNO3), to eutrophication, to tropospheric ozone formation and (in particular NO2) to irritation and damage to respiratory organs. Furthermore, NOx may react with ammonia to form secondary fine particles with negative health effects.
Formation process in combustion
Nitrogen oxides are formed by three different mechanisms during combustions. The first mechanism – the thermal formation of NOx – usually accounts for the biggest share of NOx in combustion processes with high temperatures, like the combustion of gaseous or liquid fuels. If the peak flame temperature is below 1000°C the fuel NOx mechanism accounts for the majority of the NOx emissions.
- Thermal NOx (Zeldovich mechanism) is the result of the reaction between the oxygen and the nitrogen in the combustion air:

To initially break the strong triple bond of the N2 molecules, a high temperature (above 1300°C) is required. As long as N2 and O2 are available in the combustion air and the temperature is high enough, NO is produced. Therefore, the total amount of NO is positively correlated with the flame temperature and the retention time. - Fuel NOx is the reaction product of fuel-bound nitrogen (usually N-C or N-H compounds). During combustion, these compounds react, forming ammonia (NH3) or cyanhydric acid (HCN) which then dissociate to NO. In case of lean fuel -air ratios, two thirds of the fuel-bound nitrogen is transformed to NO the rest is transformed to N2. In case of a rich (high) fuel -air ratio, less NO is formed directly. Instead more ammonia (NH3) or cyanhydric acid (HCN) is formed. If emitted to the atmosphere, NH3 and HCN decompose to NO. For this reason, the amount of NO formed by this mechanism can hardly be controlled by optimizing the combustion.
The table here after shows the content of fuel-bound nitrogen for different fuels.
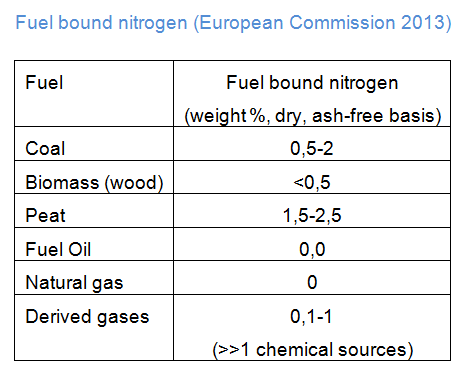
- Prompt NOx usually accounts for the smallest share of NOx and is formed in the flame front. It mainly occurs in rich fuel -air ratios. Due to the shortage of oxygen, oxidised CH-radicals are available in the combustion air. Those CH-radicals react with N2, forming cyanhydric acid that reacts to NO in the end.
The flame temperature, the retention time and the fuel -air ratio determine the amount of NOx formed during the combustion process. Therefore, the NOx emissions strongly depend on the type of fuel and the combustion technology. In general, high temperatures, a long retention time and a surplus of oxygen in the combustion air enforce the formation of nitrogen oxides.
The relationship between the combustion temperature and the NOx-formation of each path is shown in teh following table. While the positive correlation of fuel- and prompt NOx with temperature is about linear, the amount of thermal NOx is rising disproportionately fast with increasing combustion temperature.

In rich fuel-air ratios, less oxygen is available to react with the N2 in the combustion air and the fuel bound nitrogen. Therefore, both the thermal NO and fuel NO are reduced. The amount of prompt NOx, however, can be expected to rise (cf. Figure above).
The effect of the retention time also depends on the fuel-air ratio. A long retention time in rich air mixtures can reduce emissions due to the decomposition of NOx. In lean fuel-air ratios and high temperatures, however, new thermal NOx will be formed.
General approaches to reduce NOx emissions from combustion sources
In order to reduce NOx formation and NOx emissions from combustion processes different types of measures like energy efficiency improvements, fuel switch as well as primary and secondary measures are applied. To achieve the most efficient NOx reduction, beyond energy management measures, a combination of measures should be considered. To identify the best combination of measures, a site-specific evaluation is needed.
Primary measures gain to reduce the production of NOx during combustion, while secondary measures reduce the amount of already formed NOx in the flue gas. Not all measures are equally well-suited for all combustion techniques.
Fuel switching
Switching to low NOx producing fuels is one option to reduce NOx emissions but is governed by country specific conditions such as infrastructure and energy policy. Fuels with high nitrogen content like heavy fuel oil and coal may lead to high fuel NOx formation and hydrogen rich fuels like natural gas as a result of high combustion temperatures to high thermal NOx formation. The choice of the fuel may also have effects on other emissions like sulphur, particulate matter and greenhouse gas emissions as well as on applicability and need of abatement measures.
Fuel cleaning
Fuel cleaning to remove nitrogen is not a commercial option. Hydroprocessing in refineries, however, also reduces the nitrogen content of end products.
Primary Measures
Primary measures avoid or reduce the formation of NOx according to the three mechanisms described in the section ‘Formation’. Primary measures reduce NOx generation at the source by a number of different principles or methods or a combination of them:
- reducing peak temperature,
- reducing residence time at peak temperature,
- chemical reduction of NOx during combustion process,
- reducing nitrogen in the combustion process.
In the following paragraphs, an overview on available primary measures is given. Their applicability depends on the industrial sector and the production process.
Reducing peak temperature
As thermal NOx formation depends largely on the combustion temperature, a reduction in temperature is one option to reduce NOx formation. Reducing peak temperature can be achieved by the following methods i) diluting the heat produced during the combustion process, ii) cooling down, and iii) reducing the oxygen available for combustion but also by applying other combustion techniques like fluidized bed combustion (FBC) which operates at lower temperatures and includes an inherent air-staging. Main methods for reducing peak temperature are:
- substoichiometric combustion, i.e. using a fuel-rich mixture so that oxygen is a limiting factor (fuel acts also as reducing agent),
- suprastoichiometric combustion, i.e. using a fuel-lean mixture to dilute combustion heat,
- injecting cooled oxygen-depleted fuel gas to dilute combustion heat,
- injecting cooled oxygen-depleted fuel gas with added fuel to dilute combustion heat, to reduce the reaction temperature and to make oxygen a limiting factor,
- injecting water or steam to dilute combustion heat and to reduce the reaction temperature.
Reducing residence time at peak temperature
As thermal NOx formation depends largely on the time the fuel gas remains in the high temperature region, reducing this residence time reduces also NOx formation. Methods to reduce residence time include:
- injection of fuel, steam, re-circulated flue gas or combustion air immediately after combustion,
- reducing the extension of the high temperature zone which can be faster left by the flue gas then.
Chemical reduction of NOx during combustion process
NOx can be reduced to N2 using a reducing agent which is itself oxidized. The principle of chemical reduction is widely used in secondary measures but can be also used as a primary measure when reduction takes already place during the combustion process. Main methods are:
- substoichiometric combustion, i.e. in a fuel rich mixture so that the remaining fuel may act as reducing agent,
- re-burning of the flue gas with fuel added (with the added fuel acting as a reducing agent),
- generation of fuel-lean and fuel-rich conditions in the combustion zone.
Reducing nitrogen in the combustion process
Reducing NOx formation by reducing the available nitrogen can be achieved using nitrogen poor fuels like natural gas (see fuel switch) as well as by using oxygen instead of air for the combustion process.
The following primary measures which are based on the principles and methods described above are mainly in use, each with its specific advantages and disadvantages.
Some of the primary measures are typical for retrofit, others for new installations and others are only applicable in new installations.
- low excess air combustion (LEA): In order to ensure complete combustion air is often added in large excess which may result in higher thermal NOx formation when air nitrogen is oxidized. Reducing the excess air reduces also NOx formation.
- flue gas recirculation (FGR): Re-circulating cooled flue gas reduces the combustion temperature in a secondary combustion stage and also oxygen concentration so that thermal NOx formation is reduced. Heat of the flue gas may be recovered in a heat exchanger.
- air staging (AS): The principle of air staging is to create two zones, one fuel-rich zone where initial combustion takes place and a second one where air is added to ensure complete combustion. This allows reducing thermal NOx formation in the first zone where less nitrogen is available and in the second where temperature is lower. The zone may be created in different ways. In Biased Burner Firing (BBF) air and fuel flow rates are varied, in Burners Out of Service (BOOS) the fuel flow to the burner is cut for a short time and in Overfire Air (OFA) air is injected above the normal combustion zone. Staged air combustion is frequently used in conjunction with Low NOx burners (LNB).
- fuel staging (FS): Fuel staging is similar to air staging but with fuel instead of air. The first stage is extremely fuel-lean which reduces the temperature. Fuel added in the second stage acts as a reducing agent for formed NOx. In a third stage air is added to ensure burnout.
- fuel re-burning (FR): Fuel re-burning is similar to flue gas recirculation (FGR) but with added fuel in the flue gas leading to lower temperatures. If added in a second combustion stage fuel re-burning makes use of the fuel as reducing agent and is similar to fuel staging (FS).
- reduced air preheat (RAP): Combustion air is in general preheated by the flue gases to cool them down in order to improve efficiency. Reducing this preheating reduces also flame temperature and hence NOx formation but also overall energy efficiency.
- low NOx burners (LNB): Low NOx burners mix fuel and air/flue gas in a way so that different zones are created as in staged combustion. The zoning allows lower flame temperature and oxygen concentration as well as chemical reduction of NOx by fuel in some of the zones. Low NOx burners can be further differentiated into air-staged LNB, flue-gas recirculation LNB and fuel-staged LNB depending on the principle used for reducing NOx emissions. A further development is the Ultra low NOx burner.
- water/steam injection: Water and steam are injected to cool the flame and to reduce thermal NOx formation.
- oxycombustion: In oxycombustion the combustion air is replaced by oxygen so that no thermal NOx is produced. So far oxycombustion is to a larger extent only applied for glass production but its use might become more emerging in future as oxycombustion is one option to achieve high CO2 concentrations in flue gas which is an advantage for CO2 capture and sequestration.
- combustion optimisation: In combustion optimisation the combustion process is actively controlled, e.g. by making use of specialised software. One option is to slightly decrease combustion efficiency in order to reduce NOx emissions.
- catalytic combustion: Using a catalyst to reduce combustion temperature below NOx formation temperature may reduce NOx emissions very strongly. However, applications are still rare in practice though gas turbines seem to be an interesting field of application.
The techniques reported above are an inventory of available technologies to reduce NOx emissions, which does not mean that those reported technologies are applicable to each industrial sector or process of production.
Modern combustion plants usually implement a combination of different primary measures in order to reduce emissions and ensure a high efficiency.
Secondary Measures
Secondary measures or so-called end-of-pipe techniques reduce the emission of pollutants without modifying the combustion process itself. This can be obtained by converting pollutants into non-harmful products or by precipitating pollutants from the flue gas before emitting it into the environment. Modern combustions plants are often equipped with several secondary measures (dust precipitator, flue-gas desulfurization, etc.) to reduce the contents of different pollutants. The retrofitting of secondary measures usually requires additional space for utilities. Supplementary fans and heaters might be required to overcome the increased pressure drop and obtain the necessary flue gas temperatures.
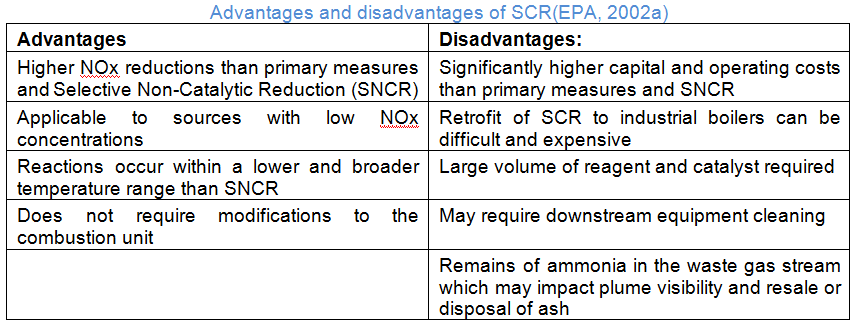
Selective Catalytic Reduction (SCR)
Selective Catalytic Reduction (SCR) is typically used for boilers between 25 and 800MW and large gas turbines and can achieve reduction rates above 90% (EPA, 2002a). In an SCR, NOx is reduced chemically by reaction with ammonia or urea in a catalytic bed. An aqueous solution of ammonia or urea is injected in the flue gas and reacts at the catalyst surface according to the following equations:
- Ammonia:
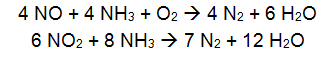
- Urea:
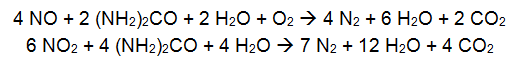
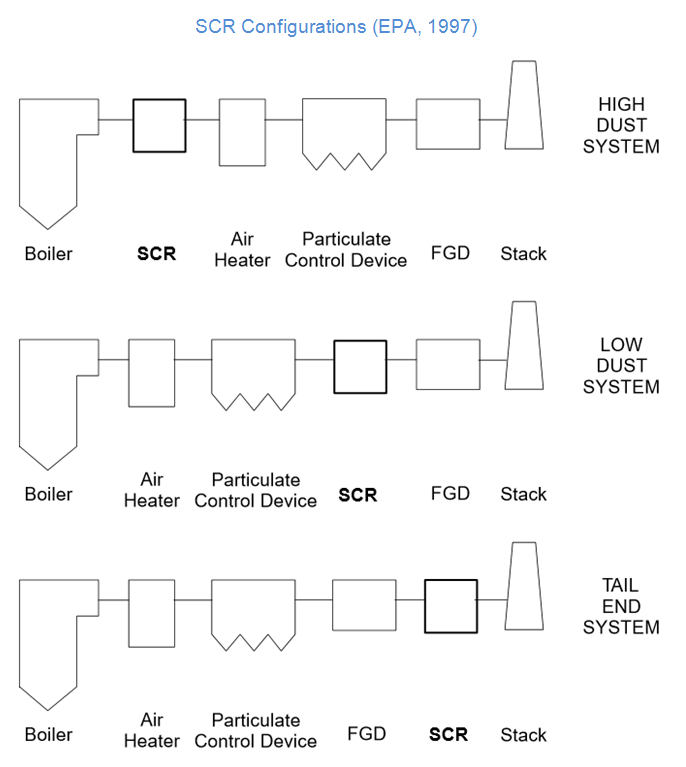
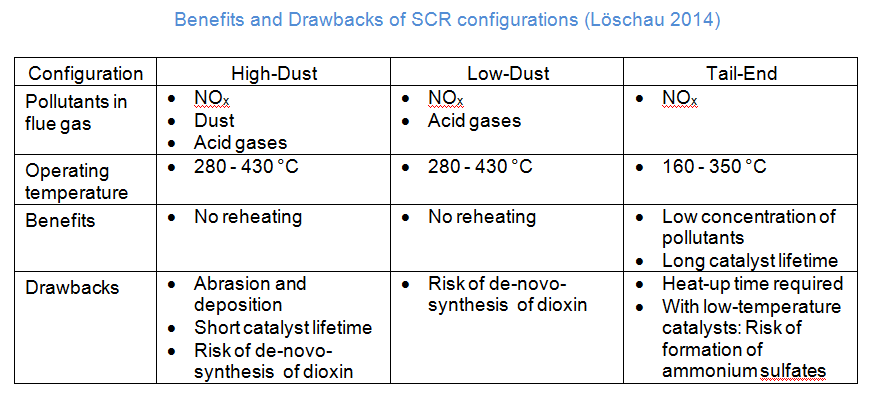
Base metal oxides, zeolites, iron oxides or activated carbon are used as catalysts. The different catalysts work at different (flue gas) temperatures between 170-510°C. The selection of the catalyst is influenced by the position of the SCR in the flue gas processing path and the presence of other pollutants in the flue gas. There are three different configurations for the integration of an SCR reactor in the flue gas treatment system (European Comission, 2013).
Configurations
The three possible configurations are shown in the following figure. The SCR is installed directly downstream the combustion and upstream the dust precipitator in the high-dust configuration. Therefore, flue-gas reheating is not necessary and the operating costs of the installation are kept relatively low. Modern catalysts have an improved resistance against abrasion and catalyst poisons. Catalyst poisons are, however, still the biggest drawback for this configuration. To overcome the drawbacks of the high-dust configuration and enhance the lifetime of the catalyst, the SCR can be installed downstream the dust precipitator in a low-dust configuration. To obtain sufficient temperatures in the SCR, special high-temperature dust-precipitators have to be installed. This can make a low-dust configuration an expensive option for retrofitting, since the dust precipitator might have to be replaced, too. The tail-end configuration, however, can be a favourable solution for existing plants. In this case, the SCR is installed as the last secondary measure downstream the dust precipitator and the flue-gas desulfurization. Compared to the other configurations, the flue gas is very clean at its entry into the SCR system and the danger of abrasion and catalyst poisoning is lower. Hence, a smaller catalyst may be sufficient, lowering the total investment. Yet, a drawback can be the necessary reheating of the flue gas. For an activated coal catalyst, a heat exchange of the raw gas with the clean gas might be sufficient, while other catalysts may require additional burners to reheat the flue gas.
Reduction Rate: 80-95%
Suitability: All fuels and combustion processes. For gas turbines a constant NH3/NOx ratio is more difficult to obtain. The ammonia slip will be higher (5-10 ppm) in order to obtain a high NOx reduction (95%).
Other Effects: Ammonia slip must be avoided due to legal regulations in many countries, but also because fly ash cannot be sold if the ammonia content exceeds a certain level (usually 2 ppm).
Installing an SCR allows to run the boiler or engine with higher NOx formation during combustion, achieving an up to 5% higher efficiency.
Ammonia slip < 5mg/Nm³
The energy consumption of an SCR is usually about 0,5% of the total electric capacity of the plant
Utilities: Ammonia can be bought and stored as granules (relevant for small utilities
Lifetime and Maintenance: The lifetime of the catalysts ranges from 40.000 to 80.000 operating hours (which is typically around 6-10 years for coal-fired units and 8-12 years for gas- and oils- fired units). Some catalysts can be regenerated up to four times, extending their lifetime up to 20 years.
Selective Non-Catalytic Reduction (SNCR)
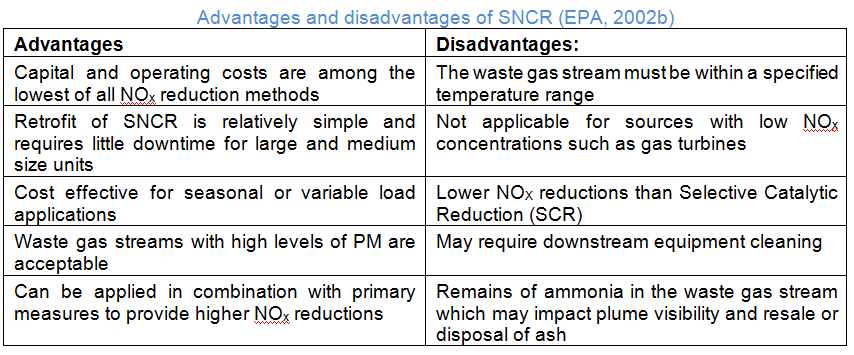
Selective Non-Catalytic Reduction (SNCR) is typically used for boilers between 5 and 600MW. It is usually less effective with low levels of uncontrolled NOx, therefore typical uncontrolled levels are between 200 and 400ppm.
SNCR is based on the reduction of NOx with injected ammonia or urea at high temperatures between approximately 900 and 1100°C. The optimal temperature to avoid unwanted reactions depends on the reagent used. Urea is non-toxic and can be stored and handled more safely. Because the urea solution is a less volatile liquid, the urea droplets can penetrate further into the flue gas, which results in better mixing. On the downside, however, urea is more expensive then ammonia.
As with load changes the temperature window in the boiler changes, several places for reagent injection might be needed (at different heights). A NH3/NOx ratio between 1,5 and 2,5 has been found to be the optimum between reduction rate and ammonia slip. Technical and legal restraints on ammonia slip are often the limiting factor for an SNCR’s reduction rate.
Example reaction with ammonia: 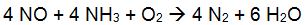
Reduction Rate: 30-50% (Some manufacturers report reductions up to 80%).
Suitability: All boilers (not for engines and gas turbines), all fuels. Not suited for boilers with big load variations and changing fuel quality.
Other Effects: Ammonia slip < 10mg/Nm³
Incomplete reaction of NH3 and NOx may result in ammonium sulphates, which can harm downstream systems. Increased NH3 concentration in flue gas and waste water.
The energy consumption of an SCR is usually about 0,1-0,3% of the total electric capacity of the plant.
Utilities: Ammonia, urea (can cause corrosion), used in smaller combustion plants <50MW)
Parameters to be considered for technical reason
The selection of the most suitable measure depends on many factors related to e.g.:
- fuels used,
- combustion technology applied,
- operational mode of installation,
- process characteristics in industrial processes,
- new installation or retrofitting,
- flue gas characteristics (NOx concentration, temperature, humidity, dust, other pollutants, catalyst poisons etc.),
- flow rate of flue gas,
- emission levels to be achieved,
- side and cross media effects,
- operational safety and reliability,
- costs.
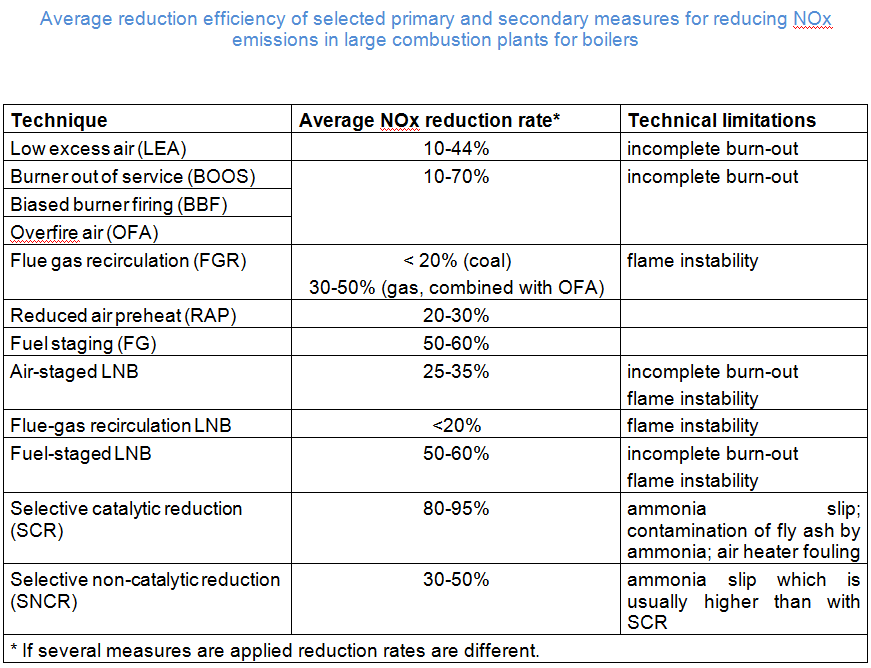
The following table gives a brief overview about the performance of primary and secondary measures for reducing NOx emissions in large combustion plants; cf. the sectoral chapters for more detailed information on sector specific issues.
Information provided by industry and accepted by the Clearing House Evaluation Committee
This section presents technological developments in reduction techniques. The documents presented were submitted to the Clearing House Committee (CHEC) by industrial or state organisations or industrial companies. There are published after agreement of the CHEC.
NOx abatement primary measures
The following document was submitted by Babcock Wanson on 30 March 2017. The CHEC agreed to publish the document on 19 October 2017.
This document addresses the technical characteristics of a primary NOx abatement technique for combustion installations using gaseous fuels. Claimed performances are 80 mg/Nm3 et 3% O2.
The following documents were submitted by Fives Pillard in February 2017. The CHEC agreed to publish these documents on 19 October 2017.
The documents address the technical characteristics of a primary NOx abatement technique (ultra low NOx burner) for combustion installations using gaseous fuels. The claimed performances are below 50 mg/Nm3 at 3% O2. The documents present performances obtained in a chemical combustion plant of 8 MW, in a 15 MW water tube boiler, in a water tube boiler of 17 MW.
