Definitions and main sources
Volatile organic compounds or VOCs means, unless otherwise specified, all organic compounds of an anthropogenic nature, other than methane, that are capable of producing photochemical oxidant by reaction with nitrogen oxides in the presence of sunlight [Gothenburg Protocol].
An organic compound is any compound containing at least the element carbon and one or more of hydrogen, halogens, oxygen, sulphur, phosphorus, silicon or nitrogen, with the exception of carbon oxides and inorganic carbonates and bicarbonates [Directive 2010/75/EU of the European Parliament and the Council on industrial emissions].
On case by case, other definitions can be encountered:
For the uses of solvent considered in technical annex VI of the [Gothenburg Protocol], volatile organic compounds (VOC) mean any organic compound as well as the fraction of creosote, having at 293.15 K, a vapour pressure of 0.01 kPa or more, or having a corresponding volatility under the particular conditions of use.
For the solvent content of products, considered in technical annex XI of the [Gothenburg Protocol], volatile organic compounds (VOCs) means any organic compound having an initial boiling point less than or equal to 250°C measured at a standard pressure of 101.3 kPa. This definition is compatible with the previous one, as there is a relation between the boiling point and the vapour pressure.
The leak detection and repair programme (LDAR) developed by the US EPA [US EPA - Protocol for equipment leak - Emission estimates EPA 453-95-017 – 1995] and standardised in Europe [EN 15446:2008 : "Fugitive and diffuse emissions of common concern to industry sectors - Measurement of fugitive emission of vapours generating from equipment and piping leaks", European committee of normalisation] is based on a vapour pressure of VOCs of 300 Pa at 295.15 K.
VOCs result from a larger number of sources both anthropogenic and natural:
- thermal processes: hydrocarbons emitted from thermal processes (fixed sources and mobile sources) contribute to the total amount of VOCs,
- use of organic solvent: an organic solvent is any VOC which is used alone or in combination with other agents, and without undergoing a chemical change, to dissolve raw materials, products or waste materials, or is used as a cleaning agent to dissolve contaminants, or as a dissolver, or as a dispersion medium, or as a viscosity adjuster, or as a surface tension adjuster, or a plasticiser, or as a preservative,
- transport and handling of liquid fuels and light organic compounds (petrol as example),
- refineries and organic chemical industries,
- natural sources.
Types of release of VOCs in the atmosphere
In order to reduce efficiently VOC emissions, it is of particular importance to consider both the reduction of stack emissions and fugitive emissions.
Stack emissions refer to emissions of which the source and the direction of gas flow is clearly definable. They enter in the atmosphere by passing through a stack or a duct designed to direct and control their flow.
Sources of fugitive emissions are not clearly defined. They enter in the atmosphere without passing through a stack or duct designed to direct or control the emissions. They include uncaptured emissions released to the outside environment via windows, doors, vents and similar openings.
In industrial plants, fugitive emissions have a diffuse character as they can arise from a lot of sources spatially dispersed.
Instead of applying emission limit values (ELV), e.g. connected to end-of-pipe measures, reduction schemes can be used. Solvent management plans have to be used as guidance for these reduction schemes. The purpose of a reduction scheme is to allow a plant operator to achieve emission reductions similar to those achieved if given limit values were to be applied by other means. Definitions of solvent management plan and of a reduction scheme are given below.
Solvent management plan and reduction scheme are a key element of annexe VI to the [Protocol to Abate Acidification, Eutrophication and Ground-level Ozone]. They help to verify compliance with given regulations, identify future reduction options, and enable the provision of information on solvent consumption, emissions and compliance with regulations to the public.
VOC emissions from anthropogenic sources can be characterised by:
- emission factors expressed in terms of mass of emitted substance (VOCs) or mass of total organic carbon per activity within a sector (e.g. g/m2 in car coating); or
- emission factors expressed in terms of mass of emitted substance (VOCs) or mass of total organic carbon per mass of solvent input (solvent purchased + solvent recovered and reused) within a sector (e.g. % of solvent used in speciality organic chemistry); or
- concentrations in terms of mass of emitted substance (VOCs) or total organic carbon per volume unit of the exhaust gases;
The importance of VOC emissions knowledge and solvent management plan
In order to minimise VOC emissions and construct a reduction plan, perfect knowledge of emissions is essential. This knowledge is based on monitoring VOC emissions in stacks, determining VOC fugitive emissions by several relevant techniques.
A solvent management plan is a key technique to understand the consumption, use and emissions of solvents, especially fugitive VOC emissions [Protocol to Abate Acidification, Eutrophication and Ground-level Ozone].
The solvent management plan consists in estimating solvent inputs and solvent outputs. Inputs are often easily known. On contrary, some outputs cannot be estimated easily. The solvent mass balance is a tool for estimating VOC emissions based on the following principles.
Definitions of inputs and outputs to be considered are as follows:
Inputs of organic solvents (I):
- I1 The quantity of organic solvents or their quantity in preparations purchased which are used as input into the process in the time frame over which the mass balance is being calculated.
- I2 The quantity of organic solvents or their quantity in preparations recovered and reused as solvent input into the process. (The recycled solvent is counted every time it is used to carry out the activity.)
Outputs of organic solvents (O):
- O1 Emissions in waste gases.
- O2 Organic solvents lost in water, if appropriate taking into account waste water treatment when calculating O5.
- O3 The quantity of organic solvents which remains as contamination or residue in products output from the process.
- O4 Uncaptured emissions of organic solvents to air. This includes the general ventilation of rooms, where air is released to the outside environment via windows, doors, vents and similar openings.
- O5 Organic solvents and/or organic compounds lost due to chemical or physical reactions (including for example those which are destroyed, e.g. by incineration or other waste gas or waste water treatments, or captured, e.g. by adsorption, as long as they are not counted under O6, O7 or O8).
- O6 Organic solvents contained in collected waste.
- O7 Organic solvents, or organic solvents contained in preparations, which are sold or are intended to be sold as a commercially valuable product.
- O8 Organic solvents contained in preparations recovered for reuse but not as input into the process, as long as not counted under O7.
- O9 Organic solvents released in other ways.
Determination of solvent consumption and NMVOC emissions can be done according to equations presented here after:
Consumption can be calculated according to the following equation:
C = I1 - O8
Total NMVOC emissions are defined as follows:
E = F + O1
Where F is the fugitive emission as defined below:
F = I1 - O1 - O5 - O6 - O7 - O8
or
F = O2 + O3 + O4 + O9
This quantity can be determined by direct measurement of the quantities. Alternatively, an equivalent calculation can be made by other means, for instance by using the capture efficiency of the process.
The fugitive emission value as well as the total emission can be expressed as a proportion of the input, which is calculated according to the following equation:
I = I1 + I2
The solvent management plan can be done on a regular basis such as an annual basis, in order to control progress carried out, take the necessary measures if deviations are observed and be in position to assess the compliance of the installation with regulation implementing ELVs.
“Guidelines for estimation and measurement of emissions of volatile organic compounds” have been developed by TFTEI. They provide guidelines to measure VOCs emissions, develop solvent management plans and calculate emissions. They correspond to the requirement in annex VI to the amended Protocol that “methods of calculation will be reflected in the guidance adopted by the Executive Body”.
General approaches to reduce VOCs emissions
For nearly all stationary sources, measures to control or to prevent VOCs emissions are available.
A distinction is generally made between primary (reduction at the source), secondary (add-on or end-of-pipe techniques) and structural measures. Unless stated otherwise, measures are applicable to new and existing installations. The reduction of VOC emissions outside of stationary sources focuses on the restrictions in the VOC content of products.
The following list gives a general outline of available measures for reducing VOC emissions, which may also be combined with secondary measures:
- More effective VOC control technologies in terms of efficient maintenance of equipment, better capture of waste gases, and generally optimized operating conditions;
- Substitution of solvent emitting VOC, e.g. use of low-organic solvent or organic-solvent-free materials and processes, such as water-based paints, water-based degreasing, etc., and/or process modifications;
- Reduction of emissions by best management practices such as good housekeeping, improved inspection and maintenance programmes, by changes in processes such as closed circuit machines, improved sealing of storage tanks, or by structural changes such as transfer of activity to locations where VOC emissions are reduced more efficiently, e.g. via pre-coating of certain products;
- Recycling and/or recovery of VOCs by control technologies such as condensation, adsorption, absorption, and membrane processes (pre-processing step). A further option is the recovery of heat (energy recovery) from VOCs. Preferably, the organic compounds should be reused on-site; this can be facilitated by the use of only few organic solvents instead of complex mixtures. Complex mixtures may be better treated off-site; however, emissions may be caused by distribution, handling, transport and storage;
- Destruction of VOCs by control technologies such as thermal or catalytic oxidation, or biological treatment. For oxidation, heat recovery is recommended in order to reduce operating costs and resource consumption. Another common procedure for destroying non-halogenated VOCs is to use VOC-laden gas streams as secondary air or fuel in existing energy-conversion units.
Primary measures
Possible primary measures for the control of emissions from the industrial use of organic solvents are: prevention (use of low- or no-organic solvent containing materials and processes), good housekeeping, process-integrated measures and structural measures. Thus, two approaches can in principle be used: a product-oriented approach, which, for instance, leads to a reformulation of the product (paints, inks, degreasing products, etc.); and process-oriented changes (increase of transfer efficiency, use of sealed chamber systems for degreasing…); Moreover, the product-oriented approach should be looked at, inter alia, because of the positive spin-off effects on emissions from the organic solvents manufacturing industry. Moreover, the environmental impact of emissions can be reduced by product reformulation to replace solvents by less harmful alternatives. Closed systems may lead to very low organic solvent emissions as well. There is a rapid ongoing development towards low-organic solvent or organic-solvent-free paints, which are among the most cost-effective solutions.
For the domestic use of paints and other solvent-containing products, only a product-oriented approach is possible. The same is true for the painting of constructions and buildings, the commercial use of cleaning products, etc. The use of water-based systems (e.g. for paints and adhesives) is an effective measure already used, especially for products for both commercial and domestic purposes.
Secondary measures
When primary measures are not sufficient to reach high VOC emission reductions or are not technically applicable, add-on control technologies can be applied alone or in combination. These techniques are used to reduce VOC emissions from processes and solvent uses.
The following techniques can be distinguished:
- techniques based on destruction of VOCs present in waste gases:
- recuperative or regenerative thermal oxidation,
- catalytic recuperative or regenerative oxidation,
- biological destruction.
- techniques enabling possible recovery of VOCs for possible reuse in the process after a specific treatment carried on site or by external companies:
- adsorption on activated carbon or zeolithe substrates,
- absorption in adapted scrubbing liquors (water, heavy oils),
- condensation and cryogenic condensation,
- membrane separation associated to other processes such as cryogenic condensation and adsorption.
Processes using thermal oxidation may enable valorisation of the energy content of VOCs. However, in most cases, this valorisation is difficult due to the low VOC concentrations generally encountered. Primary thermal energy recovery (for warming inlet gases as example) is indispensable but secondary thermal energy recovery is often most difficult to be implemented in existing plants. The VOCs concentrations have to be sufficient for enabling the oxidation unit to run without additional fuel consumption and to be consequently in autothermal conditions. Lower concentrations require additional fuel consumption which can be rapidly prohibitive.
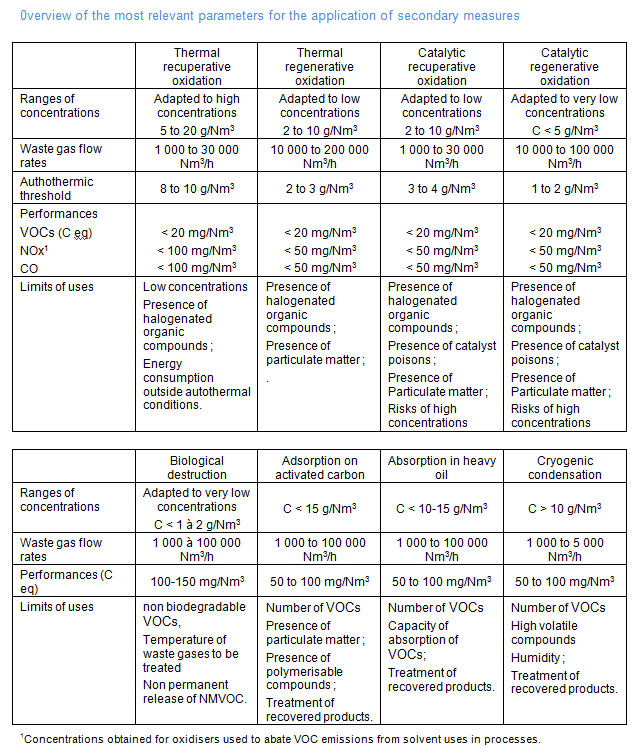
Recuperative or regenerative thermal oxidation:
In recuperative or regenerative oxidation, VOCs are destructed at high temperature. The oxidation temperature depends on the type of energy recovery system used. In recuperative oxidiser, a preheating thermal exchanger is used to heat inlet gases. The heat recovery ranges from 60 to 70 %. The temperature ranges from 650 to 750°C. The system can only be autothermal for high concentrations of VOCs ranging from 8 to 10 g/Nm3. The regenerative thermal oxidiser is constituted of two or three ceramic heat exchangers. Waste gases containing VOCs pass through a first ceramic exchanger. They are heated. They enter after in the combustion chamber maintained at about 800 to 900 °C by burners. Before being released into the atmosphere they leave the oxidiser through another ceramic exchanger, transferring its thermal energy to be re-used for preheating the next cycle. The role of the exchanger, heating or cooling, is inversed regularly. Heat recovery efficiency up to 95 % can be achieved. Regenerative thermal oxidisers are suitable for large waste gas flow rates and can be autothermal at VOCs concentrations from 2 to 3 g/Nm3. Output VOC concentrations lower than 20 mg/Nm3 can be achieved in perfectly dimensioned and operated oxidisers. Methane is largely represented in the resulting concentrations.
Recuperative or regenerative catalytic oxidation:
In recuperative or regenerative catalytic oxidation, the use of a catalyst enables VOCs to be destroyed at lower temperature than in thermal oxidation. Catalysts used are either precious metals (Platinum, Palladium or rhodium) or metal oxides (Cr, Fe, Mo, Mn, Co, Cu, Ni). The principles of heat exchange are the same as in thermal oxidation. Oxidation temperatures range from 200 to 500 °C according to catalyst used and the type of heat exchanger used. Recuperative catalytic oxidiser can be autothermal at concentrations ranging from 3 to 4 g/Nm3. Regenerative catalytic oxidiser can be autothermal at concentrations ranging from 1 to 2 g/Nm3. Life time of catalysts is limited. Lifetime of metal oxide based catalysts is about 12 000 h. Life time of precious metal based catalysts is about 15 000 h to 25 000 h. Catalysts are sensible to poisons and they can be deactivated irreversibly by certain of them. Output VOC concentrations lower than 20 mg/Nm3 can be achieved in perfectly dimensioned and operated oxidisers. When using liquid fuels with more than 0.1% S in the power plant prime mover, the catalyst lifetime can be shortened. Methane is largely represented in the resulting concentrations.
Biological destruction:
Biological destruction can be carried out in bio filters and in bio scrubbers. Micro organisms are able to destruct biodegradable VOCs in humid conditions and at low temperature. Warm waste gases (> 35 °C) must be cooled. In bio filter, microorganisms are maintained at the surface of a moist organic substrate which can be peat, heather or compost. In bio scrubbing, a combination of wet gas scrubbing and biodegradation is carried out. Microorganisms are suspended in the scrubbing water. In biofilters, residence time must be sufficient to enable biological reactions to occur. Accepted inlet VOC concentrations are low. Biological oxidation is used primarily for low concentrations. Output VOC concentrations from 100 to 150 mg/Nm3 can be achieved. Lower concentrations are however more difficult to obtain.
Adsorption on activated carbon or zeolithes:
In adsorption, VOCs are physically bound to the surface of a media which can be activated carbon or zeolithes. The adsorption capacity of activated carbon or zeolithe is limited and consequently they must be regenerated to recover their initial capacity to adsorb VOCs and recover VOC. Several configurations exist but in most of the cases, fixed bed adsorption devices are used with 2 or 3 beds. A bed is in adsorption phase, the second one is in desorption phase. Desorption is carried at high temperature with steam or inert gas. The adsorption temperature must be below 40 °C because the effectiveness of adsorption improves at low temperature. Inlet gases must be consequently conditioned. VOCs are recovered after a special treatment which involves condensation, separation and distillation if several VOCs are present. VOCs abatement efficiency depends on a lot of parameters such as adsorption temperature, type and number of VOCs to be eliminated, frequency set point for desorption. Output VOC concentrations from 50 to 100 mg/Nm3 can be achieved. Efficiencies achieved depend on numerous factors such as correct dimensioning of the installation, the frequency of desorption and the threshold value fro desorption…
Condensation and cryogenic condensation:
In condensation, VOCs are cooled below the stream dew point. Condensation of VOCs is carried out by chilling and /or pressurisation. Cooling media can be cooled water, chilled water, refrigerants and liquid nitrogen. Diverse heat exchangers equipment can be used. Condensation with cooled water, chilled water or refrigerants is often used as pre-treatment but is not sufficient to achieve high reduction of emissions. Output VOC concentrations from 100 to 150 mg/Nm3 can be achieved. Efficiencies achieved depend on numerous factors such as correct dimensioning of the installation, the frequency of desorption and the threshold value for desorption…
Liquid nitrogen is used in cryogenic (temperature less than -160°C) condensation. Cryogenic condensation is a versatile process that is not VOCs specific. Typically, condensation takes place with liquid nitrogen as the refrigerant in a straightforward heat exchange process. The VOCs condense on the shell side of the exchanger then drains into a collection tank, from which it can be recycled, reclaimed, recovered for reuse or for disposal. During condensation, the presence of water vapour or VOCs with a high melting point can cause freezing on the external surface of the tubes inside a cryogenic condenser. Special configuration exists to avoid this problem and especially a series of condenser can be used with different temperature set points. Cryogenic condensation is best suited to low waste gas flowrates and/or high VOCs concentrations. Output VOC concentrations from 50 to 100mg/Nm3 can be achieved. Efficiencies achieved depend on numerous factors such as correct dimensioning of the installation, the volatility of solvents…
Membrane separation:
VOC emissions can be concentrated using organic selective (VOCs permeable) membranes. Air and VOCs permeate through the membrane at rates determined by their relative permeabilities and the pressure difference across the membrane. Membranes are typically 10 to 100 times more permeable to VOCs than air, depending on the specific VOC characteristics. Based on the system design, the exit membrane stream VOC concentration can be increased five to fifty times the inlet membrane stream concentration. Concentrated gas streams can be then compressed and condensed by the use of conventional condensation technology. Membrane separation cannot be used alone. Subsequent gas cleaning device is necessary.
Main characteristics of techniques
The choice of a control technique will depend on various parameters, such as the concentration of VOCs in the raw gas, the gas volume flow, the type and composition of VOCs, and others. Therefore, some overlap in the fields of application may occur. In that case, the most appropriate technique must be selected according to case-specific conditions.
An overview of the most relevant parameters for the application of some secondary measures is outlined in the following table. The overall efficiency of secondary measures in the solvent-using sectors depends to a large extent on the capturing efficiency for the VOC-laden waste gas flows. Especially for fugitive emissions, capturing is paramount for the overall efficiency of the system.
Information provided by industry and accepted by the Clearing House Evaluation Committee
This section presents technological developments in reduction techniques. The documents presented in this section were submitted to the Clearing House Evaluation Committee (CHEC) by industrial or state organisations or industrial companies. There are published after agreement of the CHEC.
VOC abatement primary measures
No information submitted yet.
VOCs abatement secondary measures
The following document was submitted by the German Environment Agency (Umweltbundesamt – UBA) in spring 2017. The CHEC agreed to publish the document on 19 October 2017.
The UBA document addresses the results of a research project on best available techniques used in plants in Germany. The research project evaluated best available techniques in plants in Germany. Technical information and emission data was collected and assessed from reference plants, either applying process-integrated measures or end-of-pipe techniques for emission reduction.
The focus of the project was on the description of reference plants using:
- a biofilter system, representing a cost-effective example for reduction of VOC emissions in different solvent applications like wood coating and metals/plastics coating (see chapter 3)
- a new absorption system for VOC recovery, connected with subsequent reuse of solvents or combustion for heat recovery (see chapter 4)
Additionally, four site visits were carried out to evaluate options for emission reduction in the packaging printing sector (flexographic printing and gravure printing). Finally, cross-media effects of reducing the emission limit in publication rotogravure from 50 mg/Nm3 to 20 mg/Nm3 were documented.
In June 2015, the result of the research project was presented and discussed with the extended national expert group consisting of representatives of authorities, industry and science. The group was founded in April 2014 by the German Environment Agency (UBA) to assist in the STS BREF revision process.
